A New Kind of FDA Approval: Design Clearance
—Robert L. Read
50-Word Summary
By creating a new process for clearing tested device designs independently from manufacturing plans as a prelude to full approval to market a medical device, the FDA can allow medical devices to come to market more safely and spur cooperative innovation.
50-Word Problem
The COVID-19 pandemic created shortages of medical devices, especially PPE, which were exacerbated by the time to obtain approval to market a device, even though large communities of engineers were designing such devices. By separating design approval from manufacturing approval, this process can be made faster and therefore safer.
100-Word Outline
Dividing the current FDA approval process into two parts: 1) the clearance of the design (Design Clearance), and 2) the authorization of everyone else, allows devices to be safer and more effectively evaluated for approval. The Design Clearance creates a new kind of intellectual property asset which incentivizes cooperation on fixed-cost design independent of manufacturing which has marginal per-unit costs. This Design Clearance augments the approval process and decreases duplication of effort by both the FDA and firms producing designs. Innovations will be increased and eventually lives will be saved by adapting to the acute medical demand surges now expected.
Context and Problem
Today, the FDA grants clearance to firms to market for an intended use medical devices manufactured in specific controlled ways. In this current regulatory process, the application for clearance consists of at least these individual components:
- the design of the device itself,
- test documentation,
- the manufacturing plan for how the device will be manufactured,
- the quality system under which it is designed and manufactured,
- and the post-market surveillance.
These quality controls on manufacturing are essential to product safety.
This process makes sense in a world of relative steady demand for medical devices, in contrast to acute demand surges generated by COVID-19 or unpredictable disasters strengthened by global warming.
Traditionally, the design for a medical device was created by a firm that intended to manufacture it, and never shared with or transmitted to any other organization, for any reason. Because the design was not communicated outside the inventing organization, there was no pressing need to think of the design as separate from the manufacturing plan and other components of safety assurance. Risk associated with the design did not need to be cleanly separated from risks associated with manufacture.
However, a crisis such as a global pandemic may create a severe acute demand for a type of device which cannot be met by one firm, or even a collection of firms forming an industry. Life-threatening shortages of the devices are unaddressable in this situation and people will die.
The time and expense of bringing a device to market are daunting, even if the demand for the device is high and society is willing to devote resources to doing so. If the demand is high but acute and likely temporary, as with mechanical ventilators in spring of 2020 due to COVID-19, it may be difficult to make enough money to recoup high fixed research, development and regulatory approval costs.
Fixed design creation costs (research, development and testing) are widely treated differently for accounting and planning purposes than marginal per-unit costs of constructing and delivering devices. Yet at present, this distinction is not made or recognized in the FDA approval process.
A Proposal: Approval of Designs
Some of the effort evaluating the risks and ensuring the safety of a device is associated with the design and some other effort with the manufacturing plan. By separating these two pieces of work, the FDA may be able to more effectively evaluate applications.
The FDA should therefore create a new kind of clearance: Design Clearance (DC). This clearance should be granted through a process accepting applications that comprise a design and extensive documentation of quality assurance, risk analysis, testing and validation.
The same quality controls and risk analysis normally applied to the design and design process components of current FDA applications will be required. Emphatically, Design Clearance does not change the fact that normal FDA clearance or Emergency Use Authorization is required to market a medical device! No firm has a right to market a device manufactured from any design unless it also obtains a normal clearance/approval, of which the Design Clearance is only one part.
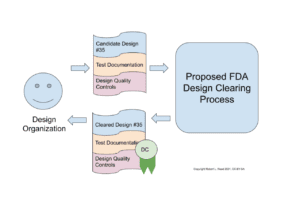
Please refer to Figure 1: Design Clearance Deliverables as I attempt to explain this.
Figure 1: Design Clearance Deliverables.
First, a Design Organization, such as Johnson & Johnson (for profit), Helpful Engineering, COSMIC, Public Invention, Open Source Medical Supplies (all non-profits), or some other firm, produces a design using the quality-controlled process that the FDA demands to assure quality, but does not develop a manufacturing plan. They produce significant test documentation of the functional prototypes of the design. They then submit these to the FDA conformant to the proposed new Design Clearing Process.
If the FDA finds the application proves the safety of the device so far as is possible examining only the design (that is, without the other processes needed to fully bring a design to market), then they may award the Design Organization a formal Design Clearance (DC). This does not give the Design Organization the right to label and market the device.
However, it may help them attract funding or investors for continued development. For example, a medical startup could use this Design Clearance to convince investors that they have an increased probability of eventually getting full approval to market. Design Clearance is therefore a valuable intellectual property asset.
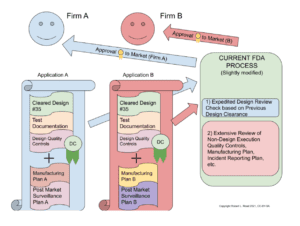
The drawing below (Figure 2) demonstrates how the Design Clearance is used in the rest of the process, to fulfill the current approval/clearance provided by the FDA.
Figure 2: Expedited Clearance/Approval Process
Let us suppose that the FDA has previously granted a Design Clearance for Design #35, including its accompanying Test Documentation and Design Quality Controls. Suppose that this design was created by an organization that shares it with two firms with each other, Firm A and Firm B, either in exchange for money or because the design was created altruistically, or both.
Now Firm A and Firm B both wish to market the device. In order to do so, they must complete the FDA application process with all of the components currently required. However, they can reuse Design #35 and its design collateral and documentation. At a minimum, they will have to augment the Design with a quality-controlled manufacturing process and a plan for post-market quality and incident surveillance. However, Firm A and Firm B may have very different approaches to manufacturing the device, perhaps performed in different facilities, using different processes, and using different materials. They may even wish to label them for different intended uses: Firm A may aim at a high-oxygen environment and Firm B may not take on the burden of assuring their materials are safe in a high-oxygen environment. The FDA must consider these two complete applications separately and independently. However, this process may be expedited by the fact that some of the work is already done and unchanged in the design which obtained Design Clearance.
Thus, Firm A and Firm B have each either saved or shared the design research, development, testing and regulatory approval cost. This is a significant fraction of their total bring-to-market cost. The FDA has shortened their overall process, or, equivalently, allowed itself more resources for the overall evaluation of the two distinct devices based on Cleared Design #35. The net result is that patients get medical devices to which more attention has been paid, assuring greater safety delivered at the same or faster overall calendar time from conception to market delivery.
In a sense, this is a minor extension of the way the FDA operates today. However, the benefits to the USA as a whole of offering Design Clearance fit our modern technical and hazard landscape well:
- The time to produce medical devices safely can be reduced.
- A firm that produces a design that is granted Design Clearance has a valuable intellectual property asset that they can sell, license, or open-source without the need to develop a manufacturing plan, thus lowering the bar for investing in new medical device design.
- Firms that choose to convey their designs have a formal mechanism that ensures their work is reusable.
- Design work funded by government grants can be usefully stockpiled in a formal way that supports emergency response.
- Non-profit organizations are incented to do a good job developing safe designs with provable safety in order to obtain Design Clearance for those designs, even if they have no intention of bringing the design to market.
Creating a Public Digital Commons of Designs
A manufacturing plan is not a very shareable, salable, or transferable object. It is probably quite specific to individual firms, corporate actors, and even manufacturing facilities. The FDA currently approves marketing, not the bare design, but the execution of complete manufacturing, handling and maintenance of a device, because all of these are necessary for safety.
However, the design of the device, if properly tested and documented, is a valuable shareable object, especially if the FDA provides Design Clearance for such devices. Ten years from now the USA could have a growing public commons of thoroughly tested medical device designs (whether cleared or not). The business of bringing devices to market would thus be lubricated by separating out expensive parts of the process so that they can be separately financed. In a sense, new business opportunities will be created and new ways of doing business established by separating out functions that are currently combined in a daunting monolith of work to bring a medical device to market. This is likely to spur life-saving innovation.
Relation to the USPRM/US Digital Stockpile Idea
Recently I collaborated with Sabrina Merlo on her proposal to the Day One Project: The USPMR/US Digital Stockpile idea. Although the Design Clearance idea presented in this paper does not depend on that idea, it synergizes with it. If the proposed USPMR/US Digital Stockpile is well-stocked with designs granted Design Clearance by the FDA, then:
- Organizations, such as small manufacturing firms, Makerspaces, and Universities, can immediately develop manufacturing plans for designs published in the USPMR/US Digital Stockpile and submit applications for clearance much more quickly than if they have to prepare an application based on a design without DC.
- The FDA saves labor processing applications based on DC-granted designs.
The USPRM/US Digital Stockpile could then contain designs whether or not they have been granted Design Clearance by the FDA. A design might start in one category and move to another as Design Clearance is granted or revoked. One can easily imagine that soon the USPRM will have designs granted DC from the FDA for personal protective equipment (PPE) such as masks, face shields, and gowns. Later, more sophisticated machines such as ventilators, PAPRs, and oxygen concentrators may be added.
**To leave feedback and comments on this paper’s Google Doc, click here**

Thanks for explaining that the FDA now plans to approve the design of devices themselves. My son has been working on a protein powder for about three years. He’s hoping to hire an FDA consultant this year to help him get the formula and design approved.
You are welcome, though of course I have done no more than introduce the concept.